Project A.13
- PhD student: Joanna Michowicz
- Supervisor: René Ketting
- Further TAC-members: Miguel Andrade , Helle Ulrich (IMB, Mainz), Dorothee Dormann (IMB, Mainz)
- Research Group
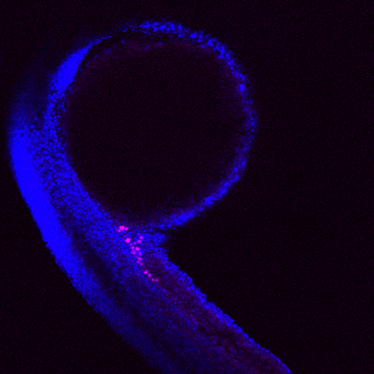
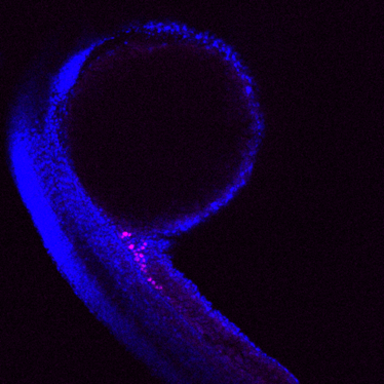
The Balbiani body (Bb) is a largely conserved, amyloid-like, phase-separated structure that has been shown in some species to be essential for producing fertile offspring. Yet, despite its aggregate properties, the Bb is a transient structure. How the disassembly of physiological amyloids occurs is unknown. Our lab recently identified promising candidates that may modulate Bb dynamics and thus subsequent germ cell development in Danio rerio. During my studies, I aim to unravel the molecular function of one of these components, the Asparagine-linked glycosylation protein 13.
Germ cells are important to ensure the continuity of species. In some vertebrates and most insects, germ cell specification is driven by maternally provided membrane-less organelles. The Balbiani body (Bb) is a large, membrane-less compartment housing organelles, germ-cell specifying transcripts and proteins. Notably, the Bb has amyloid-like features but in contrast to pathological amyloids associated with several diseases, the Bb remains dynamic. During zebrafish oogenesis Bb granules aggregate to form a single, large assembly which then fragments during oocyte maturation and subsequently following fertilisation assembles into the embryonic structure known as germ plasm. How these transitions occur and which components modulate this behaviour is largely unknown.
Intrinsically disordered proteins and nucleic acids are key players in the formation and regulation of membraneless compartments due to their multivalent nature. In Danio rerio, Bucky ball (Buc) has been shown to be required for Bb formation and generates an amyloid-like network by self-assembly of its prion-like domain. To date only Multi-Tudor-domain containing protein 6a (Tdrd6a) has been shown to modulate the phase behaviour of Buc. To determine what other proteins may be involved in modulating Bb structure, an immunoprecipitation assay using Tdrd6a followed by label-free quantitative mass spectrometry was performed. Here, multiple candidates were found to be enriched including Asparagine-linked glycosylation protein 13 (Alg13); an observation that was confirmed by additional experiments. Interestingly, in zebrafish Alg13 lacks the glycosylation domain and little is hitherto known regarding its function. Using CRISPR knockouts, transgenic techniques, imaging, purified proteins and heterologous cell culture system, I aim to characterise the role of Alg13 during germplasm formation.