Project 7.1
- PhD student: Claudia Scalera
- Supervisor: Petra Beli
- Co-Supervisors: René Ketting, Thomas Hankeln
- Further TAC-members: Vassilis Roukos
- Research Group
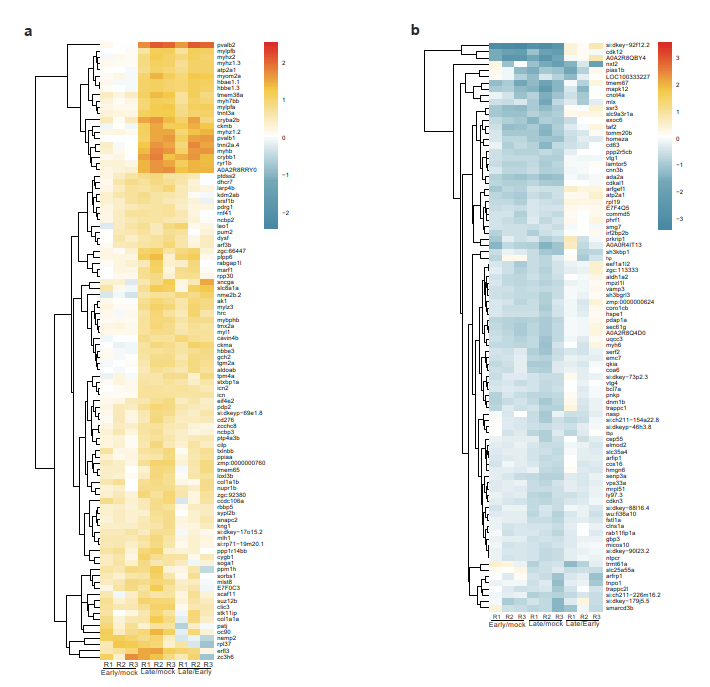
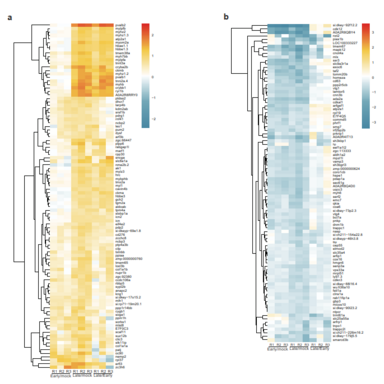
The aim of my PhD project is be to analyze the gene regulatory mechanisms that are induced upon UV stress and how these mechanisms have changed during evolution. Deeply, the project is focused on investigating modifications in gene expression and underlying protein-based molecular mechanisms that are induced after UV damage both in human and in zebrafish.
Living organisms are exposed to ultraviolet (UV) radiation from sunlight that triggers the formation of bulky photoproducts in the DNA. These UV-induced DNA lesions are repaired by the nucleotide-excision repair (NER) pathway in mammalian cells and their accumulation can lead to the onset of melanoma. Protein phosphorylation by the p38 MAP kinase pathway is a central transducer of UV-light induced DNA damage response. Moreover, exposure to UV light affects both transcriptional elongation and gene expression, which leads to enhanced DNA damage sensing and repair. In human cells p38 phosphorylates proteins complexes that control transcriptional elongation such as the negative elongation factor (NELF) complex. Indeed, phosphorylation of the NELF complex affects its protein interaction profile and leads to changes in gene expression that promote cellular survival after UV damage. Interestingly, the phosphorylated motif in the NELF complex is present in vertebrates but absent in non-vertebrates. Therefore, we would like to investigate how this pathway and the regulation of gene expression has changed in response to UV radiation during evolution. Furthermore, the importance of the phosphorylation-signaling after UV stress in Zebrafish remains unexplored to date. To this purpose, I would like to study changes in gene expression and underlying protein-based molecular mechanisms that are induced upon UV stress both in human and in Zebrafish.