Project 9.1
- PhD student: Alexander Ewerling
- Supervisor: Helen May-Simera
- Co-Supervisors: Martin Kaltenpoth, Susanne Gerber
- Research Group
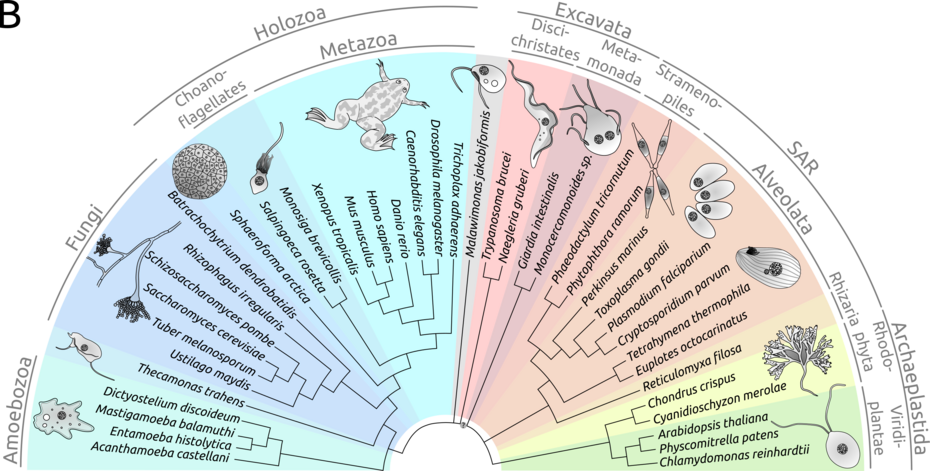
Cilia and flagella are ancestral for all eukaryotes. Although the cilium was lost on multiple occasions, ciliary proteins are conserved in eukaryotes. We propose that ciliary proteins evolved to fit non-ciliary tasks. The loss of cilia in several species, but not ciliary proteins, implies that those proteins adapted to novel functions in cells other than assembly and maintenance of cilia and flagella, leading to their involvement in regulatory processes outside a ciliary context.
Cilia and flagella are an ancestral feature of eukaryotic cells; they protrude from the cell membrane, anchored by the basal body. While conserved in their structure, motile cilia, primary cilia and flagella have evolved to fulfil different functions. Flagella, such as in spermatozoans and many single-celled protists, are responsible for locomotion by generating two-dimensional “arc-line” waveform beats (Gibbons, 1981). Motile cilia fulfil a similar function, although their movements seem to be more three-dimensional; while the motile cilia of multiciliated organisms such as Thetrahymena thermophila also serve locomotion, multiciliated cells (e.g. respiratory epithelia, ependymal cells in the ventricle) mainly use motile cilia to move fluids along a surface or epithelium (Guirao et al., 2010). Primary (or immotile) cilia differ from their motile counterparts not only in their lack of movement. Immotile cilia are solitary signalling hubs on the surface of a cell that specialise in signal perception from other cells or the outside world.
Cilia and flagella are virtually present in all eukaryotic lineages, and their proteomic make-up is highly conserved across species. In recent years ciliary proteins have been identified to function in other cellular processes possibly via a processes of co-option. We are interested to identify when and how this happened, and what additional function these cilia-associated proteins have acquired. For us it is of considerable interest if the proteins that build and maintain cilia vanished with their disappearance in some species or if they simply adapted to new tasks. In recent years, ciliary proteins have been associated with cell cycle regulation, cytoskeleton dynamics and regulatory processes in DNA damage repair and transcription factor interaction (Hua and Ferland, 2018). Loss-of-function mutations result in altered mitotic patterns, cell motility, and DNA damage responses. Nuclear localisation of ciliary proteins has been reported for human cell lines and Chlamydomonas reinhardtii (Wood et al., 2012); both of these organisms evolved a form of open mitosis. Whether there really is a correlation between functions of ciliary proteins in the nucleus and open mitosis or if this is merely a coincidence remains elusive. Studies about ciliary proteins in ciliated organisms with other forms of mitosis have not yet been conducted with regard to additional, non-ciliary functions of ciliary proteins. It remains to be uncovered if there is a correlation between extraciliary functions (especially with a nuclear localisation) and a breakdown of the nuclear envelope during mitosis.
Cilia-related proteins are still present in non-ciliated lineages of eukaryotes. Thispoints towards the development of novel functions for otherwise cilia-related proteins. Additionally, in more recent lineages of eukaryotes, we observe more open forms of mitosis. We propose that reported nuclear functions of ciliary proteins evolved alongside this phenomenon; the increasing breakdown of the nuclear envelope in more recently emerging clades of eukaryotes allowed access of ciliary proteins to otherwise restricted locations in the cell. Although the capabilities for extraciliary functions might have been present in more ancestral branches of the eukaryotic tree of life, the physical restrictions of closed mitosis might not have allowed interactions in the nucleus.