Foitzik/Baumann/Barau
Molecular manipulation of host phenotype via regulatory interference
Supervisor: Susanne Foitzik
Co-Supervisor: Peter Baumann, Joan Barau
Scientific Background
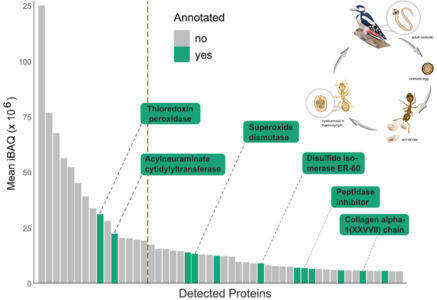
Many parasites with complex life cycles actively increase the transmission probability by altering the phenotype of their intermediate hosts. This manipulation was termed the parasites’ extended phenotype and recently even the extendedepiphenotype as parasites could alter host traits via epigenetic processes. However, the exact mechanisms by which parasites manipulate host behavior, physiology or life history traits are often still unknown. Our model is the parasitic cestode Anomotaenia brevis, whose infection strongly alters the phenotype of its intermediate host, workers of the ant Temnothorax nylanderi. Infected ants are inactive (Beros et al. 2015), have a weakly sclerotized and melanized cuticle (Scharf et al. 2012) and atrophied muscles (Feldmeyer et al. 2016). Remarkably, they show a significantly extended lifespan (Beros et al. 2021). These changes could increase transmission to the final host, a woodpecker. Our project aims to unravel the molecular mechanisms underlying these changes, including those in transcriptional activity (Stoldt et al. 2021; Feldmeyer et al. 2016, Sistermans et al. 2023), and whether they are caused by active parasitic interventions. Our recent proteomic study (Hartke et al. 2023) revealed that parasitic proteins, including antioxidants and epigenetic regulators, account for seven percent of all proteins in the hosts’ hemolymph. The genome of the parasitic cestode, which we have just completed, revealed that parasite load and transcriptionally activity is closely linked to host gene expression (Sistermans et al. 2023, in prep.).
Project description
This exciting PhD project is based on the hypothesis that the parasitic cestode Anomotaenia brevis manipulates the phenotype of its intermediate host, the ant Temnothorax nylanderi, by interfering with its gene regulation. Based on evidence of cestode proteins secreted into the host’s haemolymph, some of which with gene regulatory functions, we ask whether, how and in which tissues the parasites interfere with host gene regulation.
In particular, we will analyze whether parasite-induced shifts in host gene activity are regulated by histone modifications (CUT&TAG), DNA methylation (Whole-Genome Bisulfite Sequencing) or microRNAs. To this end, the epigenetic landscape of infected ants will be compared with that of uninfected ants, also considering the parasite load. In parallel, we will investigate the transcriptional activity of the cestodes and compare it with changes in the host. Since infected workers live more than four times as long as their uninfected nestmates, we want to uncover the epigenetic and transcriptional underpinnings of these extreme differences in life expectancy. In addition, we will use RNAi to downregulate differentially expressed host genes as well as the expression of cestode genes encoding the most abundantly secreted proteins by injecting dsRNA into the hemolymph of the ants. Since many secreted cestode proteins are not yet annotated, we aim to analyze their effects on the host phenotype, including its transcriptional activity. For their characterization we can rely on the recently generated genome of the cestode, as well as of the host, of course. Finally, we aim to analyze whether and how frequently extracellular vehicles are released by the cestode and what exactly they contain.
What you will learn
You will learn a range of cutting-edge epigenetic molecular techniques such as CUT&TAC, bisulfite sequencing for the study of DNA methylation, RNAseq, microRNA and RNAi. In addition, you will be introduced to bioinformatics analysis and over time become experienced with the development and customization of bioinformatics scripts. Furthermore, you will gain an advanced understanding of evolutionary processes, especially of parasite-host interactions. Finally, you will learn how to collect and maintain ant colonies and study their behavior, physiology and morphology.
Your qualifications
Master's degree or 4-year Bachelor's degree in biology, molecular biology or bioinformatics. Of course, good knowledge of bioinformatics, molecular genetics and evolutionary biology is needed, but if you do not yet meet all of these three criteria but are interested, please apply. Knowledge of the biology of cestodes and insects, especially social insects, is a plus, but can also be acquired during the PhD.
Publications relevant to this project
Seistrup AS, Choppin M, Govind S, Feldmeyer B, Kever M, Karaulanov E, Séguret A, Karunanithi S, Almeida MV, Ketting RF, Foitzik S. (2023) Age- and caste-independent piRNAs in the germline and miRNA profiles linked to caste and fecundity in the ant Temnothorax rugatulus. Mol Ecol. 32(22):6027-6043. DOI: 10.1111/mec.17162
Hartke, J., Ceron-Noriega, A., Marah Stoldt, M., Sistermans, T. Kever, M., Fuchs, J. Butter, F., Foitzik, S. (2023) Long live the host! Proteomic analysis reveals possible strategies for parasitic manipulation of its social host. Molecular Ecology, 32(21):5877-5889. doi.org/10.1111/mec.17155
Sistermans, T., Hartke, J., Stoldt, M., Libbrecht, R., Foitzik, S. (2023) The influence of parasite load on transcriptional activity and morphology of a cestode and its ant intermediate host. Molecular Ecology, 00, 1– 15. https://doi.org/10.1111/mec.16995
Beros, S., Lenhart, A., Scharf, I., Negroni, M.N., Menzel, F., Foitzik, S. (2021) Extreme lifespan extension in tapeworm-infected ant workers. Royal Society Open Science, 8(5):202118; DOI: 10.1098/rsos.202118
Beros S, Jongepier E, Hagemeier F, Foitzik S. (2015) The parasite’s long arm: a tapeworm parasite induces behavioural changes in uninfected group members of its social host. Proceedings of the Royal Society B, 282: 1819. doi.org/10.1098%2Frspb.2015.1473